noble gas configuration for iron|Iba pa : Baguio The next six electrons will go in the 2p orbital. The p orbital can hold up to six .
Unistrut; Fiberglass Unistrut; Fiberglass Fasteners; FNMUB - UB-050 thru UB-600 - Fiberglass Non-Metallic U-Bolts w/Hex Nuts; FNMUB - UB-050 thru UB-600 - Fiberglass Non-Metallic U-Bolts w/Hex Nuts. SKU: UB-050. $10.29) Download Fiberglass Catalog
noble gas configuration for iron,In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in .
In order to write the Calcium electron configuration we first need to know the .
Sodium (Na) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMDIba paHow to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .Magnesium (Mg) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMD

The next six electrons will go in the 2p orbital. The p orbital can hold up to six .The next six electrons will go in the 2p orbital. The p orbital can hold up to six .Because the third energy level has eight electrons and is therefore full (3s 2 3p 6) .
noble gas configuration for ironHow to Write the Electron Configuration for Oxygen. Oxygen is the eighth element . Mar 23, 2023 This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic .Using the Aufbau Principle, the Pauli Exclusion Principle, and Hund's rule to predict an atom's electron configuration using the periodic table as a .
Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. The electronic configuration of Fe 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 and Fe 3+ is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. .The electron configuration of iron is [ Ar] 3d 6 4s 2 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration .
How to write electron configurations for atoms and monatomic ions using noble gas configuration.
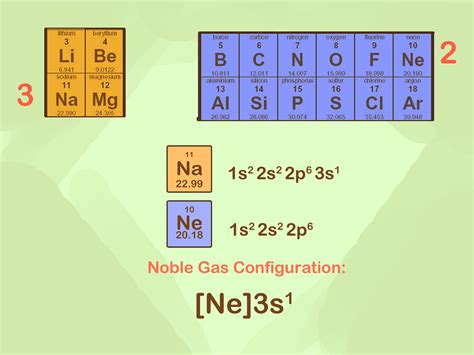
Electron Configurations. Special Cases. Electron Configurations of Ions. Anions. Cations. Contributors and Attributions. Introduction. In this section we shall assign electrons to .The Noble gas shortcut electron configuration is a way of summarizing the information about the electrons of an atom which shows only the electrons most relevant for .This method of writing configurations is called the noble gas notation, in which the noble gas in the period above the element that is being analyzed is used to denote the subshells that element has filled and after which . The electron configuration for the iron(III) ion is: "1s"^2"s"^2"2p"^6"3s"^2"3p"^6"3d"^5" The element iron, Fe, has the atomic number 26, which is the number of protons in its atomic nuclei. A neutral iron atom has 26 protons and 26 electrons. In order to form a 3^+ ion, it must lose three electrons. The ground state .They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration.The sodium ions are sodium atoms which have lost an electron, giving them the structure 1 s2 2 s2 2 p6, the same as that of the noble-gas neon. All electrons in both kinds of ions are paired. This page titled 6.5: Ions and Noble-Gas Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Ed . Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals. The noble gas before the .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Iron (Fe) [Ar] 3d 6 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2: 2, 8, 14, 2: 27: Electron configuration of Cobalt (Co) [Ar] 3d 7 4s 2: 1s 2 2s 2 2p 6 3s 2 3p .Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.
Using abbreviated electron configuration (or noble gas configuration) to identify valence electrons . In the case of Iron, the abbreviated electron configuration is [Ar] 4s 2 3d 6, so the number of valence electrons is 8 (4s 2 3d 6) Summary. Valence electrons are the outer-shell electrons of an atom. Iron has the electron configuration: [Ar]3d^(6)4s^(2) When it forms FeO the 2 outer 4s electrons are lost to form Fe^(2+) which is: [Ar]3d^(6) Iron has 8 valence electrons and its most common oxidation states are +2 and +3. In non of these does it attain a noble gas configuration. This is because is because the octet "rule" is a rule and not . Iron has 8 valence electrons and its most common oxidation states are +2 and +3. In non of these does it attain a noble gas configuration. This is because is because the octet “rule” is a rule and not a law so is not applicable in all cases. In fact it only applies to a limited number of elements.The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .
noble gas configuration for iron Iba paA Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already electron . What is the noble gas electron configuration of an iron 3 ion? Updated: 10/18/2022. Wiki User. ∙ 11y ago. . What is the noble gas configuration of Ra? The noble gas electron configuration of . Final answer: The shorthand or noble gas electron configuration for iron, Fe, is [Ar] 4s² 3d6. Explanation: The shorthand or noble gas electron configuration for iron, Fe, is [Ar] 4s² 3d6.. This means that the electron configuration of iron can be represented by the noble gas argon (Ar), with its electron configuration [Ar], followed . Like you can write 1s2 2s1 out as [He] 2s1, which basically means the electron configuration of He (the previous row's noble gas), and then whatever else is outside the square .

Noble Gas Electron Configurations quiz for 10th grade students. Find other quizzes for Chemistry and more on Quizizz for free! . What is the shorthand configuration for Iron? [Ar]3d 6 [Ar]4s 2 3d 6 [Kr]3d 6 [Kr]4s 2 3d 6. 8. Multiple Choice. Edit. 30 seconds. 1 pt. What is the shorthand configuration for Tin?
In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we.
noble gas configuration for iron|Iba pa
PH0 · noble gas notation examples
PH1 · noble gas electron configuration list
PH2 · noble gas electron configuration example
PH3 · noble gas configuration worksheet
PH4 · noble gas configuration chart
PH5 · noble gas configuration calculator
PH6 · Iba pa